Posted on: March 14, 2023
The Clinical Trials Unit (CTU) is part of the Research department, and has been conducting clinical trials at Island Health for over 20 years.
It's based in Memorial Pavillion at the Royal Jubilee Hospital in Victoria, but also conducts studies at Victoria General and Nanaimo Regional General Hospital. Our small but mightly team consists of 12 staff members who work with 20 physician investigators.
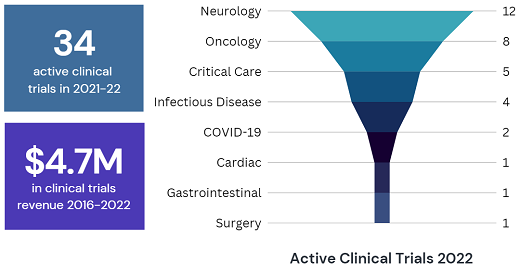
The CTU supports studies in critical care, infectious diseases, neurology, and more. These studies allow Island Health to offer novel treatments to patients and their families, and implement the latest evidence to improve care and outcomes.
For example, Island Health participated in a study that proved the efficacy of therapeutic anticoagulation for COVID-19, which has since become standard of care at Island Health for patients requiring oxygen therapy for COVID-19.
Learn More:
-
A list of active clinical trials at Island Health is available on clinicaltrials.gov
-
Clinical Trials BC seeks to maximize the health, educational, and economic benefits of clinical trials for citizens by supporting sites and developing infrastucture.
-
Learn about participating in clinical trials across Canada.
Study Opportunity: Seeking Men with Great Memories!
Currently, the CTU is participating in the Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) study: the largest study of dementia ever undertaken in Canada, which seeks to detect dementia earlier, make diagnosis easier, and predict who is at risk. This is a longitudinal observational study, meaning that information is gathered on each participant at the beginning and then research staff keep in touch with them over the coming years.
Canada, which seeks to detect dementia earlier, make diagnosis easier, and predict who is at risk. This is a longitudinal observational study, meaning that information is gathered on each participant at the beginning and then research staff keep in touch with them over the coming years.
We are looking to enroll cognitively unimpaired men to participate in the study at Royal Jubilee Hospital. You may be eligible if you are a male between 60-90 years old who has no perceived memory issues. You must also have a study partner who interacts with you at least once a week (a friend or family member), and be able to have an MRI.
If you or someone you know meets the above criteria and is interested in participating, please contact the study coordinator at 250-519-7700 x17566